VenusA-Plus four-year clinical data released: Comparable or superior to international counterparts
On April 20, Professor Liu Xianbao of the Second Affiliated Hospital Zhejiang University School of Medicine presented the four-year clinical data of VenusA-Plus, China's first retrievable transcatheter aortic valve replacement (TAVR) device, at the 10th China Valve (HangZhou).

Summary
1. VenusA-Plus provides superior safety performance: No new cardiac deaths reported; no additional safety risks ensured by retrievable delivery.
2. VenusA-Plus exhibits outstanding efficacy: Good valve function; aortic valve area, pressure gradient, and velocity all indicating sustained benefits for patients.
3. VenusA-Plus offers distinct advantages with its retrievable design: Improved procedural success rates; excellent outcomes for bicuspid aortic valve (BAV) patients.

VenusA-Plus received marketing approval from China's National Medical Products Administration (NMPA) in November 2020 as the nation's first retrievable TAVR product. This approval marked the beginning of the "retrievable era" of TAVR in China.
VenusA-Plus retains strong radial force while incorporating retrievable and repositionable features. This innovation not only reduces procedural complexity, but also drastically shortens the learning curve of doctors. Its retrievable design holds profound significance for the adoption and application of TAVR in China.
VenusA-Plus Four-year Follow-up Data
The VenusA-Plus clinical study is designed to evaluate the long-term safety and efficacy of this TAVR system. 62 patients have been enrolled for follow-up at six months, one year, two years, three years, four years and five years post-procedure. The primary endpoints include the composite rate of all-cause mortality, severe stroke, myocardial infarction, permanent pacemaker implantation, and surgical intervention within 5 years postoperatively.
The four-year follow-up data of VenusA-Plus showed zero new cases of cardiac death in comparison to the previous three-year data. In addition, there were no increases in the primary safety endpoints such as myocardial infarction, cerebral stroke, pacemaker implantation and surgical intervention. Throughout the four-year follow-up period, aortic valve hemodynamics remained normal with no moderate or severe regurgitation observed in any subject. More than 90% of subjects experienced only mild regurgitation or less, underscoring the long-term safety and efficacy of the device.
Comparable or Superior Performance to International Counterparts
Compared to the three-year follow-up data from an Evolut PRO study in the U.S., the VenusA-Plus long-term follow-up showed lower rates of major adverse events, including all-cause mortality, cardiac mortality and disabling stroke.
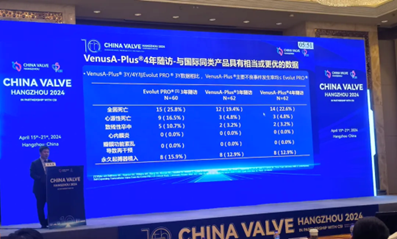
Retrievable & Non-retrievable
Analysis of patients with retrievable and non-retrievable system at four years post-procedure shows substantial improvements compared with baseline in peak velocity, peak pressure gradient, mean pressure gradient and effective aortic valve area, with sustained stability in both groups. There were no significant differences in these improvements between the groups. These results suggest that VenusA-Plus provides good safety and efficacy with retrievable features.

BAV & TAV
Subgroup analysis of patients with bicuspid aortic valve (BAV) and tricuspid aortic valve (TAV) showed substantial improvements in mean pressure gradient and aortic valve area from baseline, maintained over four years post-procedure in both groups. There were no significant differences in these outcomes between BAV and TAV subjects. These results again demonstrate the excellent safety and efficacy of VenusA-Plus in both BAV and TAV cases.
The long-term prognosis of early TAVR patients has garnered widespread attention, reinforcing the importance of long-term clinical data for precision diagnosis and treatment. With clinical follow-up extending up to 11 years, the VenusA series has accumulated extensive evidence of its sustained safety and efficacy. Looking ahead, Venus Medtech will continue its evidence-based approach, gathering clinical data and collaborating with industry leaders and peers to advance transcatheter valve therapies to new heights.

Statements
*Provided for informational and academic purposes only, this content is not intended as professional medical or legal advice. Venus Medtech makes no representations, warranties or guarantees regarding the completeness, accuracy, or timeliness of this content.
*Venus Medtech makes no representations, warranties or guarantees regarding the property or clinical performance of any medical devices mentioned.
*VENUSMEDTECH, the stylized QI logo, VenusA-Plus, etc. are trademarks of Venus Medtech (Hangzhou) Inc.
Copyright 2024. Venus Medtech(Hangzhou) Inc. All Rights Reserved.
Prev:Governor Wang Hao of Zhejiang Province visits Venus Medtech to inspect large-scale equipme···
Next:Venus Medtech scores multiple spots in Hangzhou's High-Quality Product Recommendation···